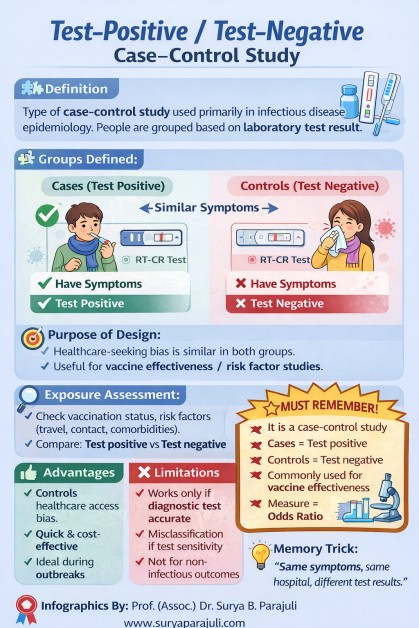
#Test-Positive / Test-Negative Case–Control Study
#🔹 Definition
A special type of case–control study.
Commonly used in infectious disease epidemiology.
Cases and controls are defined by laboratory test results, not by symptoms alone.
#🔹 Study Population
All participants:
Have similar clinical symptoms (e.g., fever, cough).
Have sought healthcare (hospital/OPD).
This ensures comparable healthcare-seeking behavior.
#🔹 Group Definition
✅ Cases (Test Positive)
Symptomatic individuals.
Laboratory test positive for the disease
(e.g., RT-PCR positive for COVID-19).
❌ Controls (Test Negative)
Symptomatic individuals.
Laboratory test negative for the same disease.
👉 Key point:
Both groups have similar symptoms; only the test result differs.
#🔹 Exposure Assessment
Done retrospectively.
Common exposures studied:
Vaccination status
Contact history
Travel history
Comorbidities
Compare exposure between:
Test-positive vs test-negative groups.
#🔹 Measure of Association
Odds Ratio (OR) is calculated.
For vaccine studies:
Vaccine Effectiveness (VE)=(1−OR)×100\text{Vaccine Effectiveness (VE)} = (1 - OR) \times 100Vaccine Effectiveness (VE)=(1−OR)×100
#🔹 Purpose of This Design
Controls healthcare-seeking bias.
Useful during outbreaks and epidemics.
Commonly used to assess:
Vaccine effectiveness
Risk factors for infection
#🔹 Advantages
Minimizes selection bias.
Cost-effective and quick.
Accurate disease classification (test-based).
Ideal for infectious diseases.
#🔹 Limitations
Depends heavily on accuracy of diagnostic tests.
Misclassification possible if test sensitivity/specificity is low.
Not suitable for non-infectious or chronic diseases.
#⭐ Must-Remember for Exams
✔ It is a case–control study
✔ Cases = Test positive
✔ Controls = Test negative
✔ Measure = Odds Ratio
✔ Commonly used for vaccine effectiveness studies
#🧠 One-Line Memory Trick
“Same symptoms, same hospital, different test results.”