#Case–Crossover Study Design – Key Points
Definition:
Observational study design used to investigate the relationship between short-term exposures and acute health events.
Each case acts as their own control, minimizing confounding from personal factors.
When to Use:
Acute outcomes: heart attack, asthma attack, seizure, accidents.
Transient exposures: physical exertion, emotional stress, caffeine, air pollution spikes.
Structure:
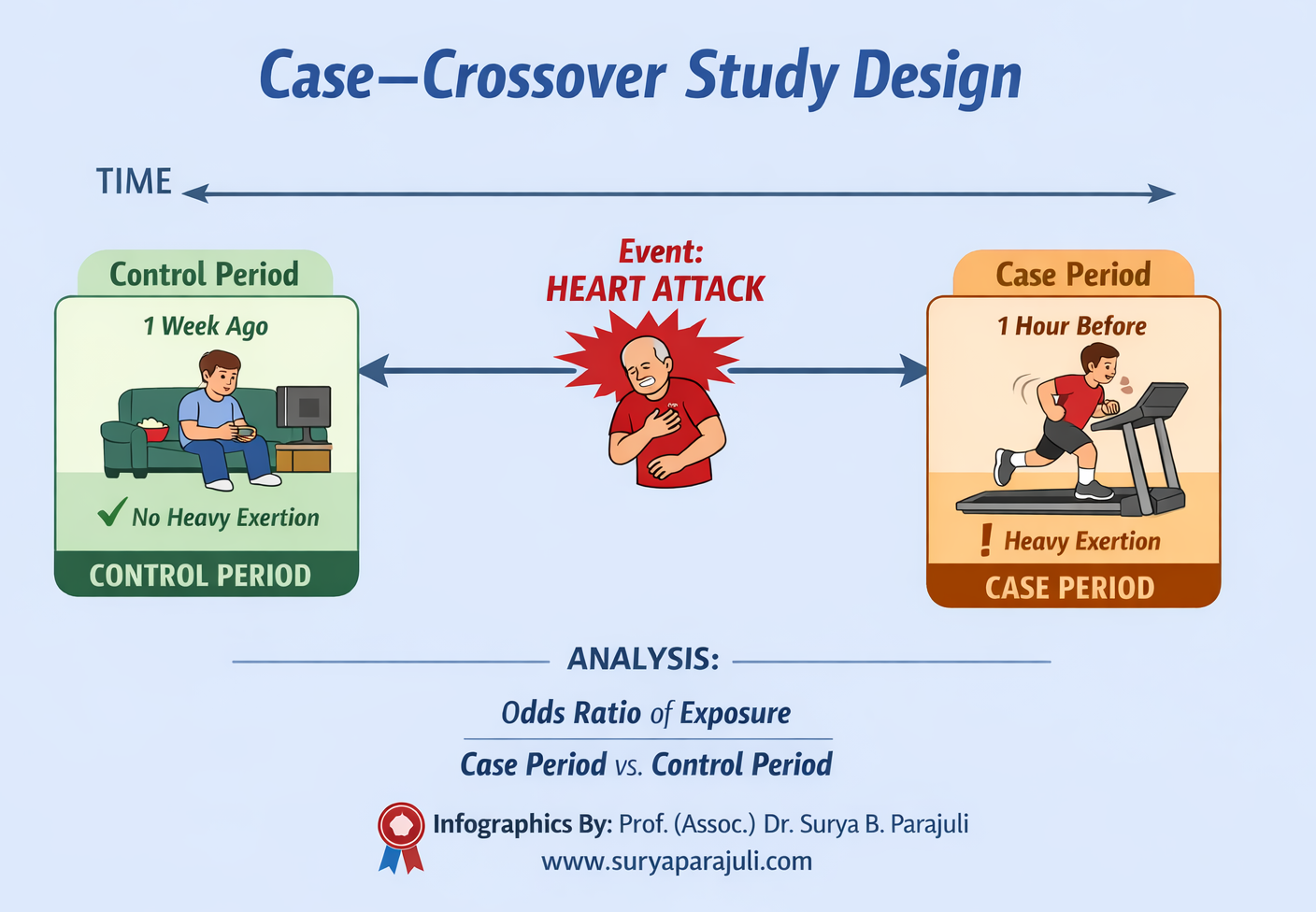
Case Period: Short time immediately before the acute event (e.g., 1 hour before heart attack).
Control Period: A comparable time without the event (e.g., same hour 1 week earlier).
Compare exposure status during case vs control periods.
Key Feature:
Self-matching: Each individual serves as their own control, reducing bias from fixed characteristics (age, gender, genetics).
Example (from infographic):
Event: Heart attack.
Exposure: Heavy physical exertion.
Observation: Patient was resting (no exertion) during control period, but exercised heavily just before heart attack.
Analysis: Odds ratio of exposure in case period vs control period to determine if exertion triggers the event.
Advantages:
Controls for stable individual characteristics automatically.
Efficient for studying rare events.
Captures short-term triggers effectively.
Limitations:
Only suitable for transient exposures and acute events.
Recall bias may occur if participants have to remember past exposures.
Not suitable for studying chronic exposures or long-term outcomes.
Take-home Message:
Think of it as a “self-controlled case study” to see if a temporary trigger caused a sudden event.
Very useful in clinical epidemiology to identify immediate risk factors for acute medical events.